 ? ? ? ? ? ? >款式描述(Style description)
? ? ? ? ? ? >款式描述(Style description)
? ? ? ? ? ? 款號:7-1AW11J \ 7-1AW12J
? ? ? ? ? ? Section number:7-1AW11J \ 7-1AW12J
? ? ? ? ? ? 衣褲分體�����,上衣連帽套頭式��,配分體褲����,褲連襪
? ? ? ? ? ? Split pants, hooded pullovers, split pants, pantyhose
? ? ? ? ? ? >細節(jié)設(shè)計(Detail design)
? ? ? ? ? ? 帽后十字交叉松緊設(shè)計、臉部細微松緊設(shè)計
? ? ? ? ? ? Cross back elastic design behind the cap, subtle elastic design on the face
? ? ? ? ? ? >配置產(chǎn)品(Configure the product)
? ? ? ? ? ? 潔凈衣袋�、潔凈口罩、滿邦鞋��、安全鞋�、
? ? ? ? ? ? Clean clothes, clean masks, Manbang shoes, safety shoes
? ? ? ? ? ? >適用場景(Applicable scene)
? ? ? ? ? ? C級�、D級
? ? ? ? ? ? C, D Grade
? ? ? ? ? ? >面料色卡(Fabric color card)
? ? ? ? ? ??
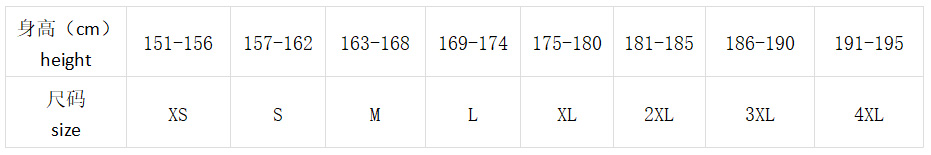
>衣服尺碼(Clothes size)
【潔凈服品名】分體式潔凈服���、二分體潔凈服、C級潔凈服�、D級潔凈防護服、D級凈化服
【潔凈服品牌】好搭檔品牌潔凈服
【潔凈服特性】潔凈工作服的質(zhì)地表面光滑�����、不產(chǎn)生靜電�,不脫落纖維和顆粒物、不吸塵�����、更衣便捷舒適
【GMP法規(guī)要求】對工作服的要求:D級潔凈區(qū)的潔凈服應當將頭發(fā)���、胡須等相關(guān)部位遮蓋�����。應當穿合適的工作服和鞋子或鞋套�����。采取適當?shù)拇胧?��,以避免帶入潔凈區(qū)外的污染物
【行業(yè)知識拓展】潔凈度級別應符合《藥品提供質(zhì)量管理規(guī)范(2010年修訂)附錄1“無菌藥品”D級潔凈區(qū)的設(shè)置要求(達到100000級)����。懸浮粒子最大允許數(shù)/立方米(塵埃粒子數(shù))只做靜態(tài)狀態(tài)測試���,不做動態(tài)狀態(tài)測試�����。
【客服咨詢】15201959939(微信同步) 15201959539(微信同步) QQ:307283910? ? ?QQ:3142699834
[ clean clothing name ] cent of this type of clean clothing, two-part clean clothing, c-clean clothing, d-clean protective clothing, d-clean clothing
[Clean clothing brand] Good partners, clean clothes
[Characteristic of clean clothing]?The surface of the clean work clothes is smooth, does not produce static electricity, does not fall off the fiber and the particulate matter, does not suck dust, changes clothes conveniently and comfortably
[GMP requirements]?The requirements of the work clothes: D Clean area clean clothing should be hair, beard and other relevant parts covered. Appropriate overalls and shoes or overshoes should be worn. Take appropriate measures to avoid the introduction of contaminants outside the clean area
[Industry Knowledge Development] The cleanliness level shall be in accordance with the requirements for setting up the D class clean area of 'sterile drugs' in appendix 1 of the 'quality control of pharmaceutical production code (revised in 2010) ' (up to level 100000) . Maximum allowable suspended particle number / cubic meter (dust particle number) only do static state test, do not do dynamic state test.